Common Nitrogen Fertilizers and Stabilizers for Corn Production
Common Nitrogen Fertilizers and Stabilizers for Corn Production
Crop Insights by Steve Butzen, DuPont Pioneer Agronomy Information Manager
Summary
- All forms of nitrogen (N) fertilizer are subject to eventual losses of N. The risk of loss depends on field and climate conditions, type of fertilizer and whether a stabilizer is used.
-
The ammonium (NH4+) form of N is quickly bound to most soils and not subject to loss in the short term. Anhydrous ammonia, urea and UAN solutions react in soil to form NH4+.
- Over time, nitrifying bacteria convert NH4+ to nitrate (NO3-) a form easily lost by leaching or denitrification. Nitrification inhibitors may reduce such losses. -
When urea or urea-ammonium nitrate (UAN) solutions are applied to the soil surface, gaseous ammonia (NH3) released by hydrolysis may be partially lost to the air.
- Urease inhibitors may reduce these volatilization losses of N. They act by blocking the active site of the urease enzyme that catalyzes the hydrolysis reaction of urea. - The use of N stabilizers is not always cost-effective; favorable returns depend on whether conditions develop that cause N losses. In that sense, N stabilizers can be thought of as insurance against the risk of N loss.
- This Crop Insights discusses anhydrous ammonia, urea, and urea-ammonium nitrate solutions, and N stabilizers that may help prevent N losses when using these fertilizers.
Introduction
N fertilizer is one of the most critical inputs in corn production. Its importance is highlighted in years when excess rainfall leads to N deficiencies and yield reductions. Under prolonged wet field conditions and warm temperatures, N can be lost from the soil (Figure 1). Losses may be moderate or severe, depending on the form of N fertilizer applied and the extent of wet, warm conditions that favor its loss.

|
| Figure 1. Flooded area of field with severe N deficiency. |
N stabilizers (also called additives) are available to help reduce N losses from the soil. These products must be used with compatible N formulations and their use should also be based on time of application and field and climate considerations to maximize their benefits. This Crop Insightsdiscusses forms of N fertilizer, conditions that favor their loss in soils, and stabilizers that may reduce those losses.
Common N Fertilizers
The most common forms of N fertilizer include anhydrous ammonia, urea, and urea-ammonium nitrate (UAN) solutions.
| Table 1. N fertilizers most commonly used for field crop production in North America. |
| Fertilizer | Form | % N |
| Anhydrous Ammonia |
Gas, applied as liquid from pressurized tank |
82% |
| Urea | Solid | 46% |
|
Urea-ammonium nitrate solutions |
Liquid | 28% - 32% |
Anhydrous ammonia, NH3, is the most basic form of N fertilizer. Ammonia, a gas at atmospheric pressure, must be compressed into a liquid for transport, storage and application. Consequently, it is applied from a pressurized tank and must be injected into the soil to prevent its escape into the air. When applied, ammonia reacts with soil water and changes to the ammonium form, NH4+. Most other common N fertilizers are derivatives of ammonia transformed by additional processing, which increases their cost. Due to its lower production costs, high N content that minimizes transportation costs and relative stability in soils, anhydrous ammonia is the most widely used source of N fertilizer for corn production in North America.
Urea is a solid fertilizer with high N content (46%) that can be easily applied to many types of crops and turf. Its ease of handling, storage and transport; convenience of application by many types of equipment; and ability to blend with other solid fertilizers has made it the most widely used source of N fertilizer in the world.
Urea is manufactured by reacting CO2 with NH3 in 2 equilibrium reactions:
2NH3 + CO2 → NH2COONH4 ammonium carbamate
NH2COONH4 → (NH2)2CO + H2O urea + water
The urea molecule has 2 amide (NH2) groups joined by a carbonyl (C=O) functional group.
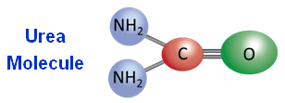
|
Urea-ammonium nitrate (UAN) solutions are also popular N fertilizers. These solutions are made by dissolving urea and ammonium nitrate (NH4NO3) in water. The composition of common N solutions is shown in Tables 2 and 3.
| Table 2. Pounds of urea and NH4NO3 in 100 lbs of UAN solution. |
| UAN-28 | UAN-30 | UAN-32 | |
| Total N Content | 28% | 30% | 32% |
| Approximate lbs in 100 lbs of solution | |||
| Urea | 30 | 32 | 35 |
| NH4NO3 | 40 | 43 | 45 |
| Water | 30 | 25 | 20 |
| Table 3. Percent of N by type in various UAN solutions. |
| UAN-28 | UAN-30 | UAN-32 | |
| Total N Content | 28% | 30% | 32% |
| Approximate lbs in 100 lbs of solution | |||
| Amide N | 14% | 15% | 16% |
| Ammonium N (NH4+) | 7% | 7.5% | 8% |
| Nitrate N (NO3-) | 7% | 7.5% | 8% |
As Table 3 indicates, 1/2 of the total N in UAN solutions is amide N (NH2-) derived from urea; 1/4 is ammonium N (NH4+) derived from ammonium nitrate, and 1/4 is nitrate N (NO3-) derived from ammonium nitrate.
Although there are several other forms of N fertilizers such as ammonium sulfate, calcium nitrate, and diammonium phosphate, over 80% of the N needs of corn in North America are met by anhydrous ammonia, urea and UAN solutions.
N Fertilizers and Soil Reactions
Anhydrous Ammonia
Anhydrous ammonia is applied by injection 6 to 8 inches below the soil surface to minimize escape of gaseous NH3 into the air. NH3 is a very hygroscopic compound and once in the soil, reacts quickly with water and changes to the ammonium (NH4+) form. As a positively charged ion, it reacts and binds with negatively charged soil constituents including clay and organic matter. Thus it is held on the soil exchange complex and is not subject to movement with water.
Soil reactions - Over time, and with appropriate soil temperatures that support biological activity, NH4+ ions are converted to the nitrate (NO3-) form by the action of specific soil bacteria in a process known as nitrification. Nitrification generally occurs at soil temperatures above 50 F, and increases as temperatures rise above this level. However, some limited activity occurs below 50 F as well. Ammonium is converted first to nitrite (NO2-) by the action of Nitrosomonas bacteria, and then to nitrate by Nitrobacter and Nitrosolobus bacteria:
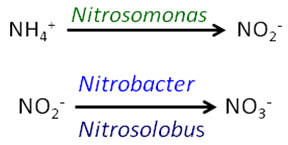
|
Only after the nitrification process has converted ammonium to negatively charged ions (that are repelled by clay and organic matter in the soil complex) can ammonium N be lost from most soils by leaching or denitrification. Plants can take up N in both the ammonium and nitrate forms. Thus, if N can be held in the ammonium form until uptake by plants, it is at little risk of loss. (Sandy soils with a very low cation exchange capacity (CEC) are an exception, as they lack enough exchange sites to bind much ammonium.)
The remaining 25% of nitrogen in UAN solutions is in the nitrate (NO3-) form. Because it is negatively charged, it will not adhere to clay and organic matter particles (which are also negatively charged) but rather, will exist as an anion in the soil solution. Because it moves with water, it is easily taken up by plant roots, but is also subject to losses by leaching and denitrification. Leaching is defined as moving below the root zone of plants; denitrification is loss of nitrate to the air as N2gas under anaerobic conditions (flooded or saturated soils).
Urea readily dissolves in water, including soil water. Thus it can be "incorporated" into the soil by sufficient rainfall or irrigation (1/2 inch is typically suggested). Otherwise, it should be incorporated by tillage to reduce losses.
Soil Reactions - If urea is applied to the soil surface and not incorporated by water or tillage, it is subject to volatilization losses of N. This occurs as urea undergoes hydrolysis to carbon dioxide and ammonia:
(NH2)2CO + H2O → CO2 + 2(NH3)
Urea hydrolysis is catalyzed by urease, an enzyme that is produced by many bacteria and some plants, and thus is ubiquitous in soils. The biological degradation of urea by urease that releases the N for plant use also makes it subject to volatilization (as NH3, a gas) depending on whether the reaction occurs in the soil or on the soil surface. If it occurs within the soil, the ammonia quickly reacts with soil water to form NH4+, which is then bound to the soil. If it occurs at the soil surface, the gaseous ammonia can easily be lost into the air. If plant residue is abundant on the soil surface, it increases bacterial populations, concentration of urease and volatilization losses of urea.
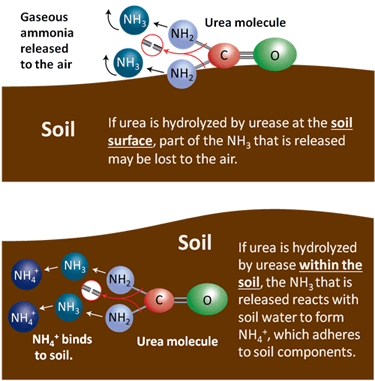
|
UAN Solutions
Urea-ammonium nitrate (UAN) solutions are mixtures of urea, ammonium nitrate, and water in various proportions. All common UAN solutions (28%, 30% and 32%) are formulated to contain 50% of actual N as amide, (from urea), 25% as ammonium (from ammonium nitrate), and 25% as nitrate (from ammonium nitrate).
Soil Reactions - The urea portion of UAN solutions reacts just as dry urea does (see previous section on urea). If applied on the surface, the amide-N in the solution may incur losses due to volatilization when urease hydrolysis releases NH3. But if UAN is incorporated by tillage or sufficient water, the NH3, quickly reacts with soil water to form NH4+. This ammonium, as well as the ammonium N derived from ammonium nitrate in the solution, adheres to soil components at the application site and is not subject to loss in the short term. Like N applied as anhydrous ammonia, this N will eventually be taken up by plants in the ammonium form, or if not, eventually converted to nitrate by soil bacteria.
The remaining 25% of N in UAN solutions is in the nitrate (NO3-) form. Because it is negatively charged, it will not adhere to clay and organic matter particles (which are also negatively charged) but rather, will exist as an anion in the soil solution. Because it moves with water, it is easily taken up by plant roots, but is also subject to losses by leaching and denitrification. Leaching is defined as moving below the root zone of plants; denitrification is loss of nitrate to the air as N2 gas under anaerobic conditions (flooded or saturated soils).
N Stabilizers / Additives
Nitrification Inhibitors
Nitrification inhibitors are compounds that slow the conversion of ammonium to nitrate, thus prolonging the period of time that N is in the "protected" form, and reducing its loss from the soil. Several compounds have proven effective for this purpose, including nitrapyrin, DCD (dicyandiamide), and ammonium thiosulfate. Of these, only nitrapyrin and DCD have current widespread use in North American agriculture.

|
| Figure 4. Nitrapyrin added to anhydrous ammonia may reduce N losses, especially if N is applied in the fall. |
| Photo courtesy of Case-IH. |
Nitrapyrin, or 2-chloro-6-(trichloromethyl) pyridine, works by inhibiting and depressing the activity of Nitrosomonas bacteria. In contrast to some other products, nitrapyrin has a bactericidal effect, actually killing part of the Nitrosomonas population in the soil. Thus it is effective until the bacterial population recovers in the zone of application and diffusion. This bacteriocidal activity is very specific to Nitrosomonas.

|
In warm soils, nitrapyrin can degrade in about 30 to 40 days. However, it is very persistent in cool soils, which contributes to its effectiveness for fall and winter applications. Measurable activity against Nitrosomonas often occurs for about 6 to 8 weeks in warm soils conducive to crop growth, and 30 weeks or more in cool soils typical of late fall and winter in the midwestern U.S. (Trenkel, 2010).
Nitrapyrin products for delaying nitrification of ammoniacal and urea fertilizers include N-Serve® 24 (launched in 1976) and Instinct® (launched in 2009). According to the product label, N-Serve 24 N stabilizer is an oil-soluble product that may be used with anhydrous ammonia, dry ammonium and urea fertilizers. When combined with a compatibility agent, N-Serve 24 may be used in the application of aqua ammonia and other liquid ammoniacal or urea fertilizer compositions. N-Serve 24 must be injected or incorporated in a zone or band in the soil with the fertilizer at a minimum depth of 2 to 4 inches during or immediately after application.
The product label for Instinct N stabilizer indicates that it is a water-based microencapsulated formulation of nitrapyrin that may be used in the application of aqua ammonia and other liquid ammoniacal or urea N fertilizer compositions such as 28%, 30% or 32% UAN. Instinct may be mixed with liquid fertilizer, insecticides, herbicides and/or water and applied as a preplant incorporated, preemergence, or postplant application. Incorporation may occur at any time up to 10 days after application and may be either by mechanical means or moisture (a minimum of 0.5 inches of rainfall or overhead irrigation).
DCD (dicyandiamide) - Following extensive use in western Europe and Japan, DCD was introduced into the U.S. in 1984, and officially approved by the EPA as a nitrification inhibitor in the late 1990s. Products containing only DCD are generally used with N solutions and liquid manure. The rate of DCD used is relative to the amount of fertilizer N applied, rather than the area of application. This may limit its practicality at very high broadcast rates of UAN solutions (e.g., > 30 gallons/acre of 28% N solution.)
In the soil, DCD has a bacteriostatic effect on Nitrosomonas, i.e., the bacteria population is not entirely killed, even with repeated applications, but its activity is suppressed or inhibited for a certain period of time (Trenkel, 2010). Depending on the amount of mineral N applied and the moisture and temperature of the soil, DCD may stabilize ammonium-N for about 4 to 10 weeks (Trenkel, 2010).
University studies have demonstrated that DCD can be effective in maintaining N in the ammonium form and increasing corn yield with both fall and spring applications. However, like other nitrification inhibitors, DCD was not always cost-effective in these studies, or significantly different than the control (no treatment).
In the U.S., products that contain DCD include Guardian® DF, Guardian-DL 31-0-0, Guardian-LP 15-0-0, Agrotain® Plus, and Super U®. Guardian-DF and Guardian-DL are intended for application with N solutions or liquid manure. In addition, Guardian DF can be impregnated on dry N fertilizers. Agrotain Plus contains a urease inhibitor as well as the DCD nitrification inhibitor, and will be discussed in a later section. Super U is a urea fertilizer with a urease inhibitor and DCD already applied; this product will be discussed in a future publication.
When to Consider Nitrification Inhibitors - The highest value of nitrification inhibitors should be realized when NO3- losses are expected to be high from leaching or denitrification, including the following conditions (Ruark, 2012):
- Tile-drained soils when leaching potential is high
- Wet or poorly drained soils
- Fields with N applied in the fall
On the other hand, nitrification inhibitors are usually least valuable when NO3- losses are unlikely, including these situations (Ruark, 2012):
- Sidedress applications, as crop demand is high at this time
- Applying on very coarse-textured soils with low CEC; if exchange sites are limited, any NH4+ions not held on the exchange can leach out of the zone containing the inhibitor
Urease Inhibitors
For the N in urea to be available to plants, it must undergo a chemical reaction that transforms the amide groups of the urea molecule to ammonia (NH3). The urease enzyme, ubiquitous in soils, catalyzes this hydrolysis reaction. If this process occurs at the soil surface, ammonia can be lost to the air. However, if this reaction is delayed until surface-applied urea is incorporated into the soil by tillage, rainfall, or irrigation, the risk of ammonia loss is greatly reduced.
Certain compounds are known to inhibit the hydrolytic action of the urease enzyme on urea and thus delay urea hydrolysis. Though many have been tested, only 1 product has been widely used in agriculture as a urease inhibitor. That product, N-butyl-thiophosphoric triamide, or NBPT, is a structural analog of urea and as such inhibits urease by blocking the active site of the enzyme. NBPT is the active ingredient in the Agrotain family of urease-inhibiting products.
Urease activity increases as temperature increases, thus hydrolysis is normally completed within 10 days at a temperature of 40 F and within 2 days at a temperature of 85 F. Hydrolysis is also highly correlated with the organic matter, total N and cation exchange capacity (CEC) of the soil; increasing as any of these factors increase.
Agrotain, with the active ingredient NBPT, is an additive for use primarily with urea (applied to urea by the retailer) and secondarily with urea-ammonium nitrate solutions. Agrotain Ultra is a more concentrated formulation of Agrotain. Agrotain or Agrotain Ultra use may be considered when urea is broadcast and not incorporated with tillage or irrigation. Research shows that N loss from surface-applied urea can be significant. The amount of loss depends on weather conditions; loss is greatest with warm, windy weather and a moist soil surface. Agrotain and Agrotain Ultra help prevent volatilization, often for 2 weeks or more, increasing the chances that rainfall will incorporate urea before losses occur.
Eventually, Agrotain and Agrotain Ultra degrade, allowing urea hydrolysis to naturally occur. This is necessary so that plants can take up and use the N from urea. However, once in the NH4+ form, this N is subject to denitrification to NO3-, a form that may be lost from the soil. Agrotain and Agrotain Ultra provide no activity against nitrifying bacteria.
Agrotain Plus is an additive specifically for UAN solutions, according to the product label. Agrotain Plus contains both the urease inhibitor NBPT and the nitrification inhibitor DCD. Thus, it acts against both the volatilization and nitrification processes that lead to N losses from UAN solutions. However, it does not protect the portion of the solution originally in the nitrate form, (i.e., the 25% of the N content of the solution derived from nitrate in ammonium nitrate).
Super U® is a urea fertilizer with the same ingredients as in Agrotain® Plus already applied. This product will be discussed in a future publication.
Performance of N Stabilizers
N stabilizers/additives have been widely tested over many years. Research results vary widely, from no advantage to yield increases of more than 20%. This is not surprising; when conditions favor N losses for a period, and an N stabilizer is applied and effective during that period, a large benefit is predictable. On the other hand, in conditions not conducive to N losses, little advantage would be expected for these products. Therefore, N stabilizers can be considered as "insurance" to help protect against N losses should conditions develop that favor those losses.
Regional performance differences for N stabilizers are expected, as soil and climate factors vary greatly across regions of North America. Soils differ by texture, drainage, organic matter, pH, slope and other variables. Climate differs by temperature extremes and durations; and rainfall amounts and patterns; as well as other variables. Because of these geographical differences, making decisions about the value of N stabilizers in each farming operation is complex. In order to make the best decisions, test results that represent your field and climate should be examined, and local prices for N fertilizers and stabilizers should be used.
Conclusions
Because the risk of N losses is always looming, growers should take appropriate precautions to reduce losses of this important crop nutrient. This can be accomplished by picking an appropriate N source and applying it near the time of crop uptake (Timing Nitrogen Applications to Corn), or using an N stabilizer when application timing is farther removed from the period of crop need.
This decision should take into account all factors that influence the risk of N loss for a particular field. These include geographic location; topography; soil type; residue level; form of N fertilizer applied; timing of application relative to crop growth; expected rainfall, temperature and soil moisture levels; and other factors. Even so, N stabilizers will not be cost effective every year, especially when conditions are not conducive for N losses. However, N stabilizers can provide insurance against the risk of N losses in many susceptible fields.
References
Butzen, S. 2011. Nitrogen application timing in corn production. Crop Insights vol. 21 no. 6. Pioneer Hi-Bred, Johnston, Iowa.
Ruark, M. 2012. Advantages and disadvantages of controlled-release fertilizers. Presentation to Wisconsin Fresh Fruit and Vegetable Conference, 1/17/2012. Dept. of Soil Science, University of Wisconsin, Madison.
Trenkel, M. 2010. Slow- and controlled-release and stabilized fertilizers: an option for enhancing nutrient use efficiency in agriculture. International Fertilizer Industry Association, Paris, France.
Read and follow all label instructions when using nitrogen inhibitors and urease inhibitors.
All products are trademarks of their manufacturers.
 中文
中文 English
English 